Safety and Regulation
Consistent disinfection under varying conditions
Water treatment and chemical automation information for swimming pools and spas has been published by many Australian Health bodies and their Fact-sheets could be found over the web. However, the following primary objectives are crucial for the safety of every recreational water environment:
-
Maintaining clean and safe water that meets the bacteriological and physiological requirements of the country/state and local Health Departments,
-
Protecting the equipment from corrosion or scaling caused by the aggressiveness of water and its constituents.
To meet with these objectives and for the convenience of our readers, we’ve gathered some important information and guidance from:
- NSW Government/ Health Protection Department
- World Health Organisation (WHO)
NSW Government, Health Protection Department
NSW Health guidance regarding Swimming pool and spa/ last published in 2013.
Public Swimming Pool and Spa Pool Advisory Document
Clause no.6.3.4. Automatic Controllers
“recently, some oversees manufacturers have developed advanced controllers which are reported to make the most of the benefits of each sensor technology and are capable of combining the inputs of both free chlorine probes, and/or redox (ORP) probes using patented control algorithms. This approach ensures both consistent disinfection under varying water conditions and reliable free chlorine residual levels”.
Clause 6.3.2 Oxidation-reduction potential
“Oxidation-reduction potential (ORP, redox) measures the rate of oxidative disinfection caused by the addition of the effects of all oxidants in the pool water. ORP is determined by using a high quality ORP probe and meter. The unit of measurement of ORP is millivolts (mV)”.
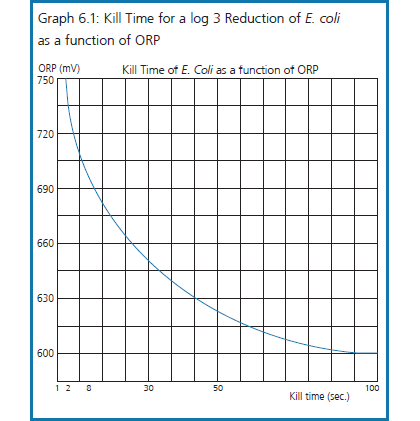
“ORP is an indicator of micro-organism inactivation. Studies on specific micro-organisms have found a direct correlation between increasing ORP and micro-organism inactivation as shown in Graph 6.1. Drinking water is adequately disinfected at an ORP of 650 mV. In swimming pools, an ORP of 700 to 720 mV allows for both a quick disinfection and for breakpoint chlorination (destruction of chloramines) where conditions permit”.
Clause 6.4 Water balancing
“The term ‘chemical water balance’ means that pH, total alkalinity and calcium hardness are within an optimum range to prevent calcium scaling, calcium corrosion and bather discomfort.
Balanced pool water prolongs the life of a pool and its fittings, assists with preventing stains and improves bather comfort”. Water balance is most significantly affected by:
- pH
- Total alkalinity
- Calcium hardness
- Temperature
Clause 6.4.2 pH
“pH is a measure of the hydrogen ion concentration in water or more simply a measure of how acid or alkaline a pool is. The pH scale ranges from 0 to 14, with 7.0 being neutral. A pH below 7.0 indicates acidic conditions and a pH above 7.0 indicates alkaline conditions.
To maintain disinfection levels and bather comfort, pH should be maintained between 7.2-7.6 (maximum 7.8). At a pH below 7.2 there is discomfort to bathers and corrosion to pool equipment. See section 4.4.1 for a discussion on the effect of pH on chlorine disinfection”.
6.4.3 Total alkalinity
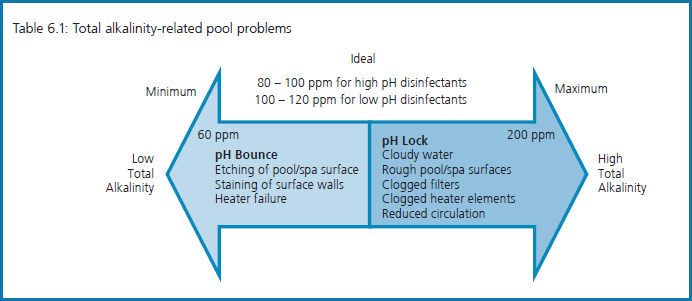
“Total alkalinity is a measure of the alkaline salts (bi-carbonates, carbonates and hydroxides) present in water. Total alkalinity prevents large fluctuations in pH, known as pH bounce. To increase total alkalinity, a buffer such as sodium bicarbonate is added to the water; to lower total alkalinity, acid is added. pH will need to be re-adjusted as a change in total alkalinity alters pH. Table 6.1 is an overview of total alkalinity levels”.
6.4.4 Calcium hardness
“Calcium hardness is a measure of the amount of dissolved calcium salts present in the water. Pool water that is very high in calcium hardness can cause scaling of heaters and pool finishes. Pools that have low calcium hardness can cause corrosion of pool equipment and etching of cement and tile grout. Some raw water sources from rural areas are naturally hard (hardness over 250 mg/L CaCO3) making these water sources more suited to treatment using sodium hypochlorite rather than calcium hypochlorite. Similarly, soft waters (hardness under 50 mg/L CaCO3) should be treated with an alkaline disinfectant such as calcium hypochlorite or sodium hypochlorite plus calcium chloride, and a gentle acid such as carbonic acid from carbon dioxide should be used to decrease pH with a gentle buffering effect. A pool consultant should be engaged to determine the most suitable pool treatment system”.
6.4.5 Temperature
“Temperature has an important effect on water balance, mainly because calcium salts become less soluble at higher temperatures. Hence high temperature pools are often subject to scaling as the calcium salts deposit on equipment and pipes. At a lower temperature the water can absorb more calcium which could cause etching of pool surfaces”.
6.4.6 Langelier saturation index
“In order to measure or to give an indication of the solubility of calcium carbonate (CaCO3) in the swimming pool water, the Langelier saturation index (LSI) is used”.
“There are four parameters that need measuring and input into a formula to calculate the index:
- pH
- Temperature – temperature factor (TF)
- Total alkalinity – alkalinity factor (AF)
- Calcium hardness – calcium factor (CF).
The LSI formula is: pH + TF + AF + CF – 12.1 = LSI where 12.1 is an adjustment constant. The ideal result for the LSI is –0.2 with a range of –0.5 to + 0.5”.
NSW Government, Health Protection Department
Disinfection of Public Swimming Pools and Spa Pools
https://www.health.nsw.gov.au/environment/factsheets/Pages/disinfection-pools.aspx
“Infections are caused by many pathogenic microorganisms simply known as germs. Disinfection is a process that kills germs, but not their spores, and reduces the risk of disease transmission. Public swimming pools and spa pool must maintain recommended disinfectant, pH and alkalinity levels to minimize the risk of disease transmission.”
Disinfection
“Disinfection is a process which kills germs, but unfortunately not their spores. The aim of disinfection is to reduce the risk of transmission of infections. Disinfection is not an instantaneous process but takes time. The higher the concentration of disinfectant the more rapidly it kills. At the minimum recommended concentrations of chlorine and bromine, the kill time is about one minute or less for most germs.
Most germs are easily controlled by disinfection but Cryptosporidium oocysts and Giardia cysts, which are types of spores, are resistant to disinfectants. Their transmission needs to be controlled by preventing these germs and their spores from entering into pool or spa. There is a separate fact sheet about Cryptosporidium and Giardia.
Disinfectant levels above the recommended minimum concentrations must be present in the pool water at all times. Disinfection must be provided by a disinfectant which leaves an effective residual in the pool that is not harmful to swimmers.”
Suitable disinfectants
“Chlorine and bromine based disinfectants are the only satisfactory pool and spa disinfectants recognized by NSW Health for use in public swimming pools and spa pools. They also oxidize and destroy some organic chemicals which may enter the pool. These disinfectants provide a readily measurable residual in the pool water. Each disinfectant has its advantages and disadvantages and pool operators should consider any disinfectant or disinfectant system carefully before use.”
Dosing of disinfectants (See separate Fact Sheet)
“All public swimming pools and spas must, as a minimum, have continuous metered dosing equipment to continuously dose the disinfectant. Automatic dosing equipment should be used it is important to respond quickly to changes in chlorine demand in busy pools. Dosing systems must be active while the pool or spa is open and for at least one hour before and after the pool is open.
Efficient chlorine pools operate on the chemical process known as breakpoint chlorination which can only be reliably and constantly achieved with dosing equipment (see separate Fact Sheet – Continuous Breakpoint Chlorination).”
Disinfection factors
- Increasing pH decreases disinfection power so that above pH 7.6, chlorination is ineffective and above pH 8.0, bromination is less effective.
- Stabilizer (cyanurate) should not exceed 50mg/L as disinfection becomes ineffective and chlorine levels should be increased if stabilizer is used. Stabilizer is not suitable for use with bromination.
- Pools should be equipped with a circulation system and filters capable of producing clean, clear water. Filtered water is easier to disinfect, reduces contaminants and produces less by-products. Circulation systems should be operated at all times while the pool or spa is open, and for at least one hour before and after the pool is open.
World Health Organisation (WHO)
Guidelines for safe recreational water environments, World Health Organisation
The World Health Organisation has published international guidelines for the safety of swimming pools and similar recreational-water environments, including standards for minimizing microbial and chemical hazards: “The oxidation–reduction potential (also known as ORP or redox) can also be used in the operational monitoring of disinfection efficacy.
In general terms for swimming pools and similar environments, levels in excess of 720 mV (measured using a silver/ silver chloride electrode) or 680 mV (using a calomel electrode) suggest that the water is in good microbial condition, although it is suggested that appropriate values should be determined on a case-by-case basis.”